Abstract
Introduction
Acute lymphoblastic leukemia (ALL) with KMT2A-rearrangement (KMT2A-r) in infants <1 year is a high-risk childhood ALL subtype, with consistently poor event-free survival (EFS) of approximately 35% when treated with intensive chemotherapy with or without hematopoietic stem cell transplant. Infant ALL blasts are characterized by DNA hypermethylation, which is hypothesized to contribute to chemoresistance by altering transcriptional regulation of gene expression. In preclinical studies of KMT2A-r blasts, epigenetic priming with DNA methyltransferase inhibitors improved the in vitro cytotoxicity of chemotherapy. Azacitidine, a pyrimidine nucleoside analog of cytidine and hypomethylating agent, has been used in combination with chemotherapy in children with leukemia. We previously reported that azacitidine was safe and well tolerated in AALL15P1 (ASPHO 2021) and herein we report survival outcomes.
Methods
The Children's Oncology Group (COG) trial AALL15P1 (NCT02828358) was a single arm, open label, groupwide pilot trial. The primary aim of the trial was to evaluate the tolerability of azacitidine in addition to Interfant-06 standard chemotherapy in infants with newly diagnosed KMT2A-r ALL. Estimation of 5-year EFS was an exploratory aim, given the small sample size. Eligibility criteria included B-ALL or acute leukemia of ambiguous lineage with ≥50% B-lymphoblasts, <366 days of age at diagnosis, and >36 weeks gestational age at enrollment. Exclusions included Down syndrome, secondary ALL, and prior cytotoxic therapy (except intrathecal chemotherapy or corticosteroids). Following an Interfant-based induction, infants with KMT2A-r received 4 courses of azacitidine, 2.5 mg/kg/dose intravenously over 10-40 minutes daily for 5 consecutive days in each course, immediately preceding the start of a chemotherapy course on day 6. Infants without KMT2A-r were removed from protocol following induction and did not receive azacitidine. Treatment failure was defined as failure to achieve M1 marrow status (<5% blasts by morphology) with resolution of extramedullary leukemia by the end of consolidation. EFS and overall survival (OS) were measured from the time of enrollment.
Results
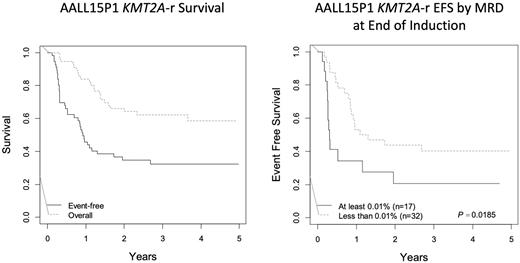
The study accrued from March 2017 to December 2019 and all protocol-directed treatment concluded in December 2021. Of the 78 infants enrolled, 56 had KMT2A-r (72%), and 53 completed induction therapy and received at least 1 course of azacitidine. No patients were ineligible. Diagnostic clinical characteristics of infants with KMT2A-r included four infants age <7 days (7%), 13 age <90 days (23%), 18 with white blood cell count ≥300,000/µL (32%), 32 CNS2 (57%), and four CNS3 (7%). As of the data cutoff (06/30/2022), the median follow-up is 3.8 years, and the 3-year EFS (SE) and OS (SE) rates are 34.2% (+/- 0.08) and 63.8% (+/-0.08), respectively, for infants with KMT2A-r. Six infants experienced treatment failure. Minimal residual disease (MRD) levels of marrow blasts by flow cytometry in COG-approved laboratories were submitted for 49 KMT2A-r patients at the end of induction. Of those, 32 were MRD negative <0.01% (65%), eight MRD positive 0.01%-<1% (16%) and nine MRD positive ≥1% (18%). Event-free survival was significantly associated with MRD; the 3-year EFS of patients with any positive MRD was 20.6% (+/-0.13) vs. 40.1% (+/-0.09) (p=0.0185) for those without MRD. As previously reported, at no time did the trial meet or exceed the pre-determined dose limiting toxicity boundaries, and the rates and types of toxicities observed were within the expected range for infants receiving standard chemotherapy alone.
Conclusions
Epigenetic priming with azacitidine prior to standard chemotherapy was well tolerated in infants with KMT2A-r ALL, but the EFS was consistent with the poor survival of historical outcomes. Positive flow MRD at the end of induction predicted a higher risk of treatment failure, relapse, or death, in comparison to negative MRD, but EFS was still unacceptably low for MRD-negative patients. There remains an urgent need for improved therapies for infants with KMT2A-r ALL.
Disclosures
Guest:Jazz Pharmaceuticals: Speakers Bureau; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Gore:Sanofi-Paris: Current equity holder in publicly-traded company; Onkure: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Dura: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Millennium: Research Funding; Takeda Development Center Americas, Inc.: Research Funding; Amgen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Mirati: Current equity holder in publicly-traded company. Raetz:Pfizer: Research Funding; BMS: Other: Data and Safety Monitoring Board. Hunger:Servier: Honoraria; Jazz: Honoraria; Amgen: Current equity holder in private company, Honoraria. Brown:Bristol Myers Squibb: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal